Abstract
Background Patients with intermediate-, high-, or very-high-risk myelodysplastic syndrome (I/H/vHR-MDS) need durable treatment options with favorable tolerability. Sabatolimab (MBG453) is a novel immunotherapy targeting TIM-3, an immuno-myeloid regulator expressed on immune and leukemic stem cells, but not on normal hematopoietic stem cells. Patients with higher-risk MDS experience poor outcomes and limited treatment options. HMAs are approved for the frontline management of higher-risk MDS, but 50% of HMA-treated patients experience primary failure, and most responders progress within 2 years. Novel therapies that provide improved durable outcomes with favorable safety profiles are needed for these patients. In a phase Ib study (NCT03066648), sabatolimab+HMAs demonstrated an encouraging safety-tolerability profile and emerging durable responses in patients with higher-risk MDS (64% overall response rate and approximately 84% of patients still in response after 6 months). Sabatolimab is being studied in multiple phase I-III trials within the STIMULUS program. We present the STIMULUS MDS-US trial, designed to further assess the safety and efficacy of sabatolimab+HMAs, including an oral HMA.
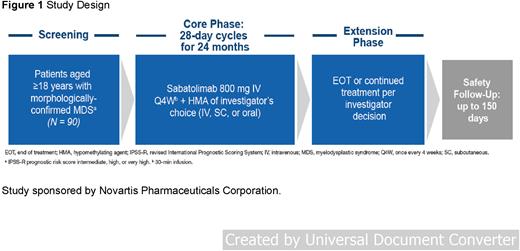
Study Design and Methods: STIMULUS MDS-US (NCT04878432) is a nonrandomized, single-arm, open-label, phase II study evaluating the safety and efficacy of sabatolimab in combination with FDA-approved HMAs of the investigator's choice in patients with MDS in the United States. A 12-month extension phase will continue investigating safety and efficacy after 24 months of treatment. The planned enrollment is estimated to be 90 patients. The trial is currently enrolling. Eligible patients are aged ≥18 years and have treatment-naive, higher-risk MDS (IPSS-R intermediate- or high/very high-risk) not suitable for intensive chemotherapy or hematopoietic stem cell transplant. Patients will receive IV sabatolimab 800 mg on day 8 (D8; or once between D5-8 to coincide with partner HMA administration) of each 28-day cycle for 24 months plus the investigator's choice of the following HMAs: oral decitabine (Inqovi®, decitabine 35 mg and cedazuridine 100 mg, D1-5); IV decitabine (20 mg/m2, D1-5); or IV or subcutaneous azacitidine (75 mg/m2, D1-7 or D1-5+8-9). Primary end points are incidence and severity of adverse events (AEs) and serious AEs. Secondary endpoints are complete remission (CR) rate (according to International Working Group for the Prognosis for MDS), progression-free survival, overall survival, leukemia-free survival, percentage of patients with CR, marrow CR and/or partial remission, duration of CR, time to CR, and percentage of patients with improvement in red blood cell/platelet transfusion independence.
Disclosures
Zeidan:Celgene/BMS, Novartis, AbbVie, Gilead, Kura, Loxo Oncology, Geron: Other: Clinical Trial Committee; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie: Consultancy, Honoraria, Other: Advisory Board; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie, Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Astex, Pfizer, Medimmune/AstraZeneca, ADC Therapeutics: Research Funding; Gilead, Kura, Loxo Oncology: Consultancy, Honoraria, Other: Clinical Trial Committee; Novartis, Cardiff Oncology, Pfizer: Other: Travel Support; Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Gilead, Kura, Loxo Oncology, Otsuka, Jazz, Agios, Acceleron, Astellas, Daiichi-Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Ionis, Epizyme, Janssen, Syndax, Genentec: Consultancy, Honoraria, Other: Advisory Boards; Jazz, Agios, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, Beyondspring, Gilead, Kura, Tyme, Janssen, Syndax, Geron, Ionis, Epizyme: Consultancy, Honoraria; Astex, Medimmune, Astrazeneca, ADC Therapeutics: Research Funding; Celgene/BMS, AbbVie, Pfizer, Boeringer-Ingelheim, Trovagene, Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, Amgen, Otsuka: Consultancy, Honoraria, Research Funding. DeZern:GERON: Other: DSMB; Novartis: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Syntrix Pharmaceuticals: Research Funding; CTI BioPharma: Consultancy, Honoraria. Borate:Takeda: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; AbbVie/Genentech: Membership on an entity's Board of Directors or advisory committees. Ide:Novartis: Current Employment. Sabo:Novartis: Current Employment. Marques Ramos:Novartis Pharma AG: Current Employment. Sun:Novartis: Current Employment. Lyons:Incyte Corporation: Consultancy, Other: Research Consulting Services; Texas Oncology/The US Oncology Network: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Other: Leadership; Celgene: Honoraria. Garcia-Manero:Acceleron Pharma: Consultancy; Curis: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Aprea: Honoraria; Gilead Sciences: Research Funding; AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal